cheilanthoids
cheilanthoid ferns
Carl Rothfels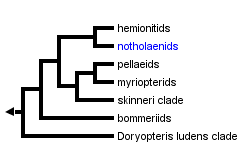

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Cheilanthoids are a diverse clade most notable for their ability to colonize xeric habitats, including deserts of the New and Old World, as well as rocky habitats worldwide. These are generally smallish ferns (large individuals may be over a meter in length, but that is unusual) with leaves that are densely scaly or hairy (many Cheilanthes, Astrolepis, Bommeria), covered beneath with a wax-like flavonoid deposit (“farina”; Notholaena, Argyrochosma, Pentagramma), or protected with a thick cuticle (Pellaea; Doryopteris).




Left: Fertile leaf of Cheilanthes lendigera, abaxial surface, showing pronounced false indusia and hairy indument. © 2008 Amanda Grusz. Center: When water-stressed, Notholaena standleyi leaves roll inwards, exposing their farina-covered under-surfaces. © Carl Rothfels. Right: The leaves of Pellaea truncata are protected with a thick cuticle. © Carl Rothfels
The cheilanthoid clade includes approximately 20 genera (as currently circumscribed) and 500 species. Generic concepts within the group have been particularly challenging, inducing Rolla and Alice Tyron to pronounce cheilanthoids the “most contentious group of ferns with respect to a practical and natural generic classification” (Tryon and Tryon, 1982). Much of the challenge of developing stable morphology-based classifications appears to be a result of convergent evolution driven by adaptation to xeric habitats (Gastony and Rollo, 1998; Kirkpatrick, 2007; Lellinger, 1989; Prado et al., 2007; Rothfels et al., 2008; Tryon and Tryon, 1973; Windham et al., 2008)—there seems to be a limited number of morphologies that allow ferns to survive and reproduce in extremely xeric conditions (basically the hairy, waxy, and thick-leaved morphologies mentioned above), and cheilanthoid ferns have explored each of them multiple times independently.
Characteristics
Cheilanthoids show a great variety of form, both in terms of gross morphology (e.g., leaf shape) and in specifics of their reproductive structures (e.g., position of the sporangia, presence or absence of a false indusium). These latter characters, in particular, have often been used as the basis for delimiting cheilanthoid genera, but are increasingly shown to be homoplasious (and not indicative of evolutionary relationships).
Almost all of the “farinose” ferns—those that have the undersurfaces of their leaves covered with a dense layer of wax-like deposits—are cheilanthoids, including members of the genera Argyrochosma, Cheilanthes, Notholaena, and Pentagramma. Outside of the cheilanthoids this feature is rare, with Pityrogramma (in the pteridoids) being the main exception. Farina can be composed of different compounds, with certain compounds or combinations of compounds being distinctive for particular groups of ferns (Wollenweber, 1984; Wollenweber and Schneider, 2000). See A Quick Guide to Farinose Ferns.
Discussion of Phylogenetic Relationships
Of the primary subclades of Pteridaceae, the cheilanthoids are the least well supported. This apparent lack of support is due to some uncertainty about the position of what we here interpret to be the earliest-diverging cheilanthoid branch (the Doryopteris ludens clade). While the remaining taxa are strongly supported as monophyletic, and the Doryopteris ludens clade has never been resolved in a position other than that presented here (as sister to the rest of the cheilanthoids), this placement is rarely strongly supported (Schuettpelz et al., 2007; Zhang et al., 2005).
The remainder of the cheilanthoids fall into six strongly supported primary clades that we treat (following Windham et al., 2008) as the: bommeriids; skinneri clade; myriopterids; pellaeids; notholaenids; and hemionitids.
References
Gastony, G. J., and D. R. Rollo. 1995. Phylogeny and generic circumscriptions of cheilanthoid ferns (Pteridaceae: Cheilanthoideae) inferred from rbcL nucleotide sequences. American Fern Journal 85:341-360.
Kirkpatrick, R. E. B. 2007. Investigating the monophyly of Pellaea (Pteridaceae) in the context of a phylogenetic analysis of cheilanthoid ferns. Systematic Botany 32:504-518.
Lellinger, D. B. 1989. The ferns and fern-allies of Costa Rica, Panama, and the Chocó (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A:1-364.
Prado, J., C. D. N. Rodrigues, A. Salatino, and M. L. F. Salatino. 2007. Phylogenetic relationships among Pteridaceae, including Brazilian species, inferred from rbcL sequences. Taxon 56:355-368.
Rothfels, C. J., M. D. Windham, A. L. Grusz, G. J. Gastony, and K. M. Pryer. 2008. Toward a monophyletic Notholaena (Pteridaceae): Resolving patterns of evolutionary convergence in xeric-adapted ferns Taxon 57:712-724.
Schuettpelz, E., H. Schneider, L. Huiet, M. D. Windham, and K. M. Pryer. 2007. A molecular phylogeny of the fern family Pteridaceae: assessing overall relationships and the affinities of previously unsampled genera. Molecular Phylogenetics and Evolution 44:1172-1185.
Tryon, R. M., and A. F. Tryon. 1973. Geography, spores, and evolutionary relations in the cheilanthoid ferns. Pages 145-153 in The phylogeny and classification of ferns. (A. C. Jermy, J. A. Crabbe, and B. A. Thomas, eds.). Academic Press, New York.
Tryon, R. M., and A. F. Tryon. 1982. Ferns and Allied Plants with Special Reference to Tropical America. Springer-Verlag, New York.
Windham, M. D., L. Huiet, E. Schuettpelz, C. J. Rothfels, J. Beck, A. L. Grusz, G. Yatskievych, and K. M. Pryer. 2008. Using plastid and nuclear DNA sequences to redraw generic boundaries and demystify species complexes in cheilanthoid ferns. American Fern Journal (in review).
Wollenweber, E. 1984. Exudate flavonoids of Mexican ferns as chemotaxonomic markers. Rev. Latinoamer. Quim. 15:3-11.
Wollenweber, E., and H. Schneider. 2000. Lipophilic exudates of Pteridaceae -- chemistry and chemotaxonomy. Biochemical Systematics and Ecology 28:751-777.
Zhang, G., X. Zhang, and Z. Chen. 2005. Phylogeny of cryptogrammoid ferns and related taxa based on rbcL sequences. Nordic Journal of Botany 23:485-493.
Title Illustrations

| Scientific Name | Doryopteris ludens |
|---|---|
| Location | Cultivation, Miami, Florida, USA |
| Acknowledgements | Image reproduced with permission from PlantSystematics.org |
| Specimen Condition | Live Specimen |
| Identified By | R. Moran |
| Copyright | © 2005 |
| Scientific Name | Adiantopsis radiata |
|---|---|
| Location | Cultivation |
| Acknowledgements | Image reproduced with permission from PlantSystematics.org. |
| Specimen Condition | Live Specimen |
| Identified By | R. Moran |
| Copyright | © 2005 |
| Scientific Name | Cheilanthes skinneri |
|---|---|
| Location | Costa Rica, Prov. de Guanacaste, Palo Verde National Park |
| Specimen Condition | Live Specimen |
| Identified By | C.J.Rothfels |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Carl Rothfels

|
About This Page
Carl Rothfels

Duke University, Durham, North Carolina, USA
Correspondence regarding this page should be directed to Carl Rothfels at
Page copyright © 2008 Carl Rothfels
 Page: Tree of Life
cheilanthoids. cheilanthoid ferns.
Authored by
Carl Rothfels.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
cheilanthoids. cheilanthoid ferns.
Authored by
Carl Rothfels.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 23 December 2008
- Content changed 23 December 2008
Citing this page:
Rothfels, Carl. 2008. cheilanthoids. cheilanthoid ferns. Version 23 December 2008 (under construction). http://tolweb.org/cheilanthoids/133070/2008.12.23 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site