Sigmodontinae
Neotropical mice and rats
Scott J. Steppan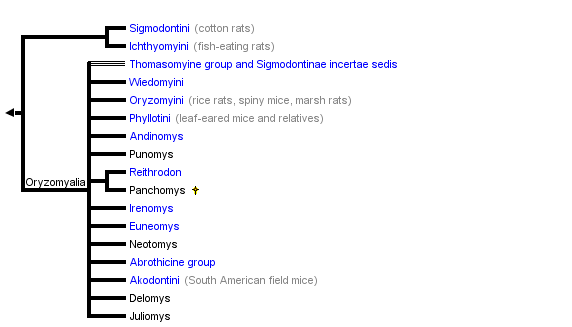

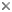
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxTree adapted primarily from Dickerman (1992) and Steppan (1995).
Introduction
The sigmodontines comprise one of the greatest recent radiations of mammals. There are currently more than 300 species occupying a wide variety of niches: from hopping mice to semi-aquatic swimmers that hunt fish and crustaceans. Other habitats and behaviors include arboreal and burrowing species, grass eating specialists, insectivores, and omnivores. They can be found from sea level to over 5000 meters (16,400 ft.) high in the Andes. Estimates for the age of this group range from 4.5 to 15 million years. There is a strong consensus that the lineage originated in North America, but there is controversy over whether their greatest diversification occurred in North or South America. If the former, then a remarkable pattern of numerous invasions must have taken place. If the later, then they might constitute a large scale adaptive radiation. In either case, they figure prominantly in the Great American Faunal Interchange. The centers of diversity are now in South America, but many species can be found in Central America and two or three species occur as far north as southern USA. Some of the more commonly known species include the cotton rats (Sigmodon), rice rats (Oryzomys), marsh rats (Holochilus), South American field mice (Akodon), leaf-eared mice (Phyllotis), and vesper mice (Calomys). Although the majority of species avoid human contact, a few species have become agricultural pests. Others are vector for human diseases, such as Argentine hemoragic fever, which is carried in some Calomys. Because of their abundance in the wild and museum collections, sigmodontines have have figured prominantly in ecological and multivariate evolutionary studies.
Characteristics
Sigmodontines are stereotypic mice and rats, but as might be expected in such a large group, there are many variations on the theme. Like all murids, the dental formula is 1/1 0/0 0/0 3/3 (one pair of incisors and three pairs of molars, both upper and lower). The skeleton is relatively generalized. Sigmodontines all possess complex penises, with a few well defined exceptions. A complex penis has two lateral horns on the cartilagenous distal baculum, making it appear trident shaped (Fig. 3). The predominantly North American Neotominae (often subsumed within Sigmodontinae) lack these lateral horns and are characterzied as simple-penised forms. Although still subject to debate, most systematists assume that the complex penis is ancestral. Few other characters define this diverse array of species, and there are few diagnostic dental features that can be used to definitively assign fossils, which are frequently only represented by teeth or lower jaws. Possible synapomorphies for the subfamily from Steppan (1995) are: a complex penis, the entoglossal process of the hyoid is absent, a dual articulation of the first rib with the seventh cervical and first thoracic vertebrae, the fifth metatarsal does not extend posterior to the cuboid/calcaneum articulation, the entepicondylar foramen of the humerus is absent while the supertrochlear foramen is present, and a gap is present between the trochlear process and articular facet of the calcanuem.

Figure 2. Generalized diagram of a sigmodontine molar, from Reig (1986).
Discussion of Phylogenetic Relationships
The above tree summarizes the available phylogenies, drawing mostly from nuclear DNA studies of Steppan et al. (2004 and unpubl. data), Jansa and Weksler (2004), D'Elia (2003), Weksler (2003), DNA hybridization study of Dickerman (1992), mitochondrial DNA phylogenies of Engel et al. (1998) and Smith and Patton (1999), and some from the morphological cladistic analysis of Steppan (1995). Earlier studies of Karyotypes (Gerdner and Patton, 1976), phallic morphology (Hooper and Musser, 1964), and synthetic studies (Hershkovitz, 1962; Reig, 1980; 1986) have provided a foudation for the later studies.
Sigmodontini is placed at the base of the sigmodontines based primarily on a variety of molecular studies (immunological, mtDNA sequence, DNA hybridization). Morphological data tend to place Sigmodontini with Phyllotini or closely related to it, a result that is probably due to convergence on simplified dentitions and the long branch leading to Sigmodon, the sole member of Sigmodontini.
The Ichthyomyini, the fish-eating rats, are also placed as an early branch, although there is less direct evidence to support the arrangment. Voss' (1988) supposition that Ichthyomyini is not closely related to any other sigmodontine group is supported by DNA data (Dickerman, 1992; Jansa and Weksler 2004; Steppan et al. unpubl. data). Steppan (1995) placed them in the tetralophodont tribal group (lacking a complete mesoloph and thus with four rows of cusps on the molars rather than the plesiomorphic five rows). Support from morphological characters was weak and supported primarily by the simplification of the molars, a commonly repeated trend in rodents.
The Thomasomyine group, conventionally treated as a tribe or subsumed within Oryzomyini, appears to be paraphyletic and incudes several basal lineages once the Oryzomyini sensu stricto are defined and removed.
Akodontini conventionally included the abrothicine group but not the oxymycterines or scapteromyine. Work by Smith and Patton (1999) using the mitochondrial gene cytochrome b and by D'Elia (2003) and Steppan et al. (unpubl. data) using multiple nuclear and mitochondrial genes however support the arrangment shown above.
The Phyllotini had been taxonomically stable for a long time and morphologists were largely confident about its close relationship to the Akodontini. However, mtDNA phylogenies do not support monophyly (Engel et al., 1998; Smith and Patton, 1999) and in particular Smith and Patton (1999) found that it included several genera of no clear affinities. The nuclear data sets confirm and extend those findings, hence the proliferation of "unique lineages" (Smith and Patton, 1999) on the tree with no tribal affiliation.
Geographic Distribution

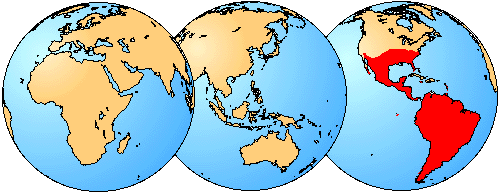
Figure 3. Although several species of sigmodontines can be found as far north as the central United States, the large majority of species are restricted to South America or the southern portion of Central America.
References
Arata, A. 1964. The anatomy and taxonomic significance of the male accessory reproductive glands of muroid rodents. Bull. Fla. St. Mus., Biol. Sci. 9:1-42.
Baker, R. J., B. F. Koop, and M. W. Haiduk. 1983. Resolving systematic relationships with G-bands: A study of five genera of South American cricetine rodents. Syst. Zool. 32:403-416.
Baskin, J. A. 1986. The late Miocene radiation of Neotropical sigmodontine rodents in North America. 3:287-303.
Carleton, M. D. 1973. A survey of gross stomach morphology in New World Cricetinae (Rodentia, Muroidea), with comments on functional interpretations. Misc. Publ.,Mus. Zool, Univ. Michigan 146:1-43.
Carleton, M. D. 1980. Phylogenetic relationships on neotomine-peromyscine rodents (Muroidea) and a reappraisal of the dichotomy within New World Cricetinae. Misc. Publ. Mus. Zool., Univ. Mich. 157:1-146.
Carleton, M. D., and G. G. Musser. 1984. Muroid rodents. 289-379 in Orders and Families of Recent Mammals of the World (S. Anderson and J. K. Jones Jr. eds.). John Wiley and Sons, New York.
Catzeflis, F. M., A. W. Dickerman, J. Michaux, and J. A. W. Kirsch. 1993. DNA hybridization and rodent phylogeny. 159-172 in Mammalian Phylogeny: Placentals (F. S. Szalay, M. J. Novacek and M. C. McKenna eds.). Springer-Verlag, New York.
Czaplewski, N. J. 1987. Sigmodont rodents (Mammalia; Muroidea; Sigmodontinae) from the Pliocene (early Blancan) Verde Formation, Arizona. J. Vert. Paleont. 7:183-199.
D'El?a, G. 2000. Comments on recent advances in understanding sigmodontine phylogeny and evolution. Mast. Neotrop. 7:47-54.
D'El?a, G. 2003. Phylogenetics of sigmodontinae (Rodentia, Muroidea, Cricetidae), with special reference to the akodont group, and with additional comments on historical biogeography. Cladistics 19:307-323.
Dickerman, A. W. 1992. Molecular systematics of some New World muroid rodents. Ph.D. dissertation, University of Wisconsin-Madison.
Engel, S. R., K. M. Hogan, J. F. Taylor, and S. K. Davis. 1998. Molecular systematics and paleobiogeography of the South American sigmodontine rodents. Mol. Bio. Evol. 15:35-49.
Gardner, A. L., and J. L. Patton. 1976. Karyotypic variation in oryzomyine rodents (Cricetinae) with comments on chromosomal evolution in the neotropical cricetine complex. Occ. Pap. Mus. Zool., La. State Univ. 49:1-48.
Gyldenstolpe, N. 1932. A manual of Neotropical sigmodont rodents. Kungl. Svenka Vetenskapsakad. Handl. Ser. 3:1-164.
Hershkovitz, P. 1955. South American marsh rats, genus Holochilus, with a summary of sigmodont rodents. Fieldiana: Zool. 37:639-673.
Hershkovitz, P. 1962. Evolution of neotropical cricetine rodents (Muridae) with special reference to the phyllotine group. Fieldiana: Zool. 46:1-524.
Hershkovitz, P. 1966. South American swamp and fossorial rats of the Scapteromyine group (Cricetinae, Muridae) with comments on the glans penis in murid taxonomy. Z. Saugetierkd. 31:81-149.
Hooper, E. T., and G. G. Musser. 1964. The glans penis in neotropical cricetines (Muridae) with comments on classification of muroid rodents. Misc. Publ. Mus. Zool., Univ. Mich. 123:1-57.
Jacobs, L. L., and E. H. Lindsay. 1984. Holarctic radiation of Neogene muroid rodents and the origin of South American cricetids. J. Vert. Paleo. 4:265-272.
Jansa, S. A., and M. Weksler. 2004. Phylogeny of muroid rodents: relationships within and among major lineages as determined by IRBP gene sequences. Mol. Phylogenet. Evol. 31:256-276.
Marquet, P. A. 1989. Paleobiogeography of South American cricetid rodents: a critique to Caviedes & Iriarte. Rev. Chi. Hist. Nat. 62:197-197.
Musser, G. M., and M. D. Carleton. 1993. Family Muridae. 501-756 in Mammal species of the world: a taxonomic and geographic reference (D. E. Wilson and D. M. Reeder eds.). Smithsonian Institution, Washington.
Reig, O. A. 1980. A new fossil genus of South American cricetid rodents allied to Wiedomys, with an assessment of the Sigmodontinae. J. Zool. Lond 192:257-281.
Reig, O. A. 1986. Diversity patterns and differentiation of high Andean rodents. 404-439 in High altitude tropical biogeography (F. Vuilleumier and M. Monasterio eds.). Oxford University Press, London.
Reig, O. A. 1987. An assessment of the systematics and evolution of the Akodontini, with the description of new fossil species of Akodon (Cricetidae, Sigmodontinae). Fieldiana: Zool., n.s. 39:347-399.
Sarich, V. 1985. Rodent macromolecular systematics. 423-452 in Evolutionary relationships among rodents: a multidisciplinary analysis. (W. P. Luckett and J.-L. Hartenberger eds.). Springer-Verlag, Berlin.
Simpson, G. G. 1980. Splendid isolation. The curious history of South American mammals. Yale University Press, New Haven.
Slaughter, B. H., and J. E. Ubelaker. 1984. Relationships of South American cricetines to rodents of North America and the Old World. J. Vert. Paleo. 42:255-264.
Smith, M. F., and J. L. Patton. 1993. The diversification of South American murid rodents: evidence from mitochondrial DNA sequence data for the akodontine tribe. Biol. J. Linn. Soc. 50:149-177.
Smith, M. F., and J. L. Patton. 1999. Phylogenetic relationships and the radiation of sigmodontine rodents in South America: evidence from cytochrome b. J. Mamm. Evol., 6:89-128.
Steppan, S. J. 1995. Revision of the leaf-eared mice Phyllotini (Rodentia: Sigmodontinae) with a phylogenetic hypothesis for the Sigmodontinae. Fieldiana: Zool. 80:1-112.
Steppan, S. J., R. M. Adkins, and J. Anderson. 2004. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst. Biol. 53:533-553.
Vorontsov, N. N. 1959. The system of hamster (Cricetinae) in the sphere of the world fauna and their phylogenetic relations [in Russian]. Biul. Mosk. Obsh. Isp. Prir. Otd. Biol. 64:134-137.
Voss, R. S. 1988. Systematics and ecology of ichthyomyine rodents (Muroidea): patterns of morphological evolution in a small adaptive radiation. Bull. Am. Mus. Nat. Hist. 188:259-493.
Voss, R. S., and M. D. Carleton. 1993. A new genus for Hesperomys molitor Winge and Holochilus magnus Hershkovitz, with comments on phylogenetic relationships and oryzomyine monophyly. Am. Mus. Novitates 3085:1-39.
Voss, R. S., and A. V. Linzey. 1981. Comparative gross morphology of male accessory glands among neotropical Muridae (Mammalia: Rodentia) with comments on systematic implications. Misc. Publ. Mus. Zool., Univ. Michigan 159:1-39.
Weksler, M. 2003. Phylogeny of Neotropical oryzomyine rodents (Muridae : Sigmodontinae) based on the nuclear IRBP exon. 29:331-349.
About This Page
Scott J. Steppan

Florida State University, Tallahassee, Florida, USA
Correspondence regarding this page should be directed to Scott J. Steppan at
Page copyright © 1996 Scott J. Steppan
All Rights Reserved.
Citing this page:
Steppan, Scott J. 1996. Sigmodontinae. Neotropical mice and rats. Version 01 January 1996 (under construction). http://tolweb.org/Sigmodontinae/16548/1996.01.01 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site