Monotomidae
Root-eating beetles
Thomas McElrath, Yves Bousquet, and Joseph V. McHugh

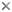
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Monotomidae is a family of small (1.5-6.0mm) predaceous or mycophagous cucujoid beetles. There are currently 35 genera with 247 described species, divided into two subfamilies. Rhizophaginae contains the Holoarctic and Oriental genus Rhizophagus Herbst (53 spp.). The subfamiliy Monotominae is divided into five tribes: Lenacini (Lenax), Monotomini (Monotoma), Thionini (Thione, Shoguna, Arunus), †Rhizophtomini (†Rhizophtoma) and Europini for the remaining 28 genera.
DISTRIBUTION
Monotoma Herbst (40 spp.) has a cosmopolitan distribution. Thione Sharp (5 spp.) has a disjunct Neotropical and Australian distribution, while its sister genus Shoguna Lewis (7 spp.) has a predominantly Oriental distribution, but includes species on Christmas Island, Madagascar, and Japan. The monotypic Lenax mirandus Sharp is restricted to New Zealand.
The Europini, containing the vast majority of monotomid diversity, have a cosmopolitan distribution. Europs Wollaston (49 spp.) is widely distributed in both the Old and New World, and Bactridium LeConte (28 spp.) is widely distributed in the Nearctic and Neotropical regions but it is likely that neither is monophyletic (Bousquet 2010). Mimemodes Reitter (15 spp.) is distributed in the Oriental, Australian, and eastern Palearctic regions. Hesperobaenus LeConte (10 spp.) and Leptipsius Casey (6 spp.) are predominantly New World genera, but a species of the former has been described from Tahiti (Bousquet 2010). The remaining genera are mostly monotypic with limited distribution.
BIOLOGY AND ECOLOGY
Adults of many genera (e.g. Rhizophagus, Lenax, Malinica, Monotomopsis, Tarunius, Renuka, Leptipsius, Europs, Aneurops, Maceurops, Shoguna) are often collected under the bark of dead and fungus-infested trees (Sen Gupta 1988). Adults of Rhizophagus, Mimemodes, and Shoguna can be found in the galleries of scolytine bark beetles (Sen Gupta 1988, Kishi 1970). Lawrence (1984) suggested that the Thionini occurring in scolytine galleries feed on the various species of ambrosia fungi in the tunnels. Species of the genus Monotoma often occur in decaying vegetable matter, including man-made habitats such as compost heaps and haystacks, as well as stored products. Species in the subgenus Monotoma (Gyrocecis) are inquilines in nests of Formica ants in North America and Europe (Bousquet and Laplante 2000). Monotoma hoffmanni inhabits the refuse piles of Atta ants (Hinton & Ancona 1935). Monotoma arida Casey has been collected from nest debris of Neotoma spp. Mimemodes spp. have been collected from haystacks and flowers of Boga medula in India (Sen Gupta 1988). Hesperobaenus spp. are associated with yucca and sotol plants in the Southwestern US (Bousquet 2002) and are often collected under the bark of Quercus spp. in Eastern North America. Phyconomus marinus (LeConte) and Monotoma producta LeConte are found only on the intertidal zones of sandy beaches on the Pacific and Atlantic Coasts of North America (Doyen 1976, Bousquet 1999, Chandler 1983). The European Rhizophagus parallelocollis Gyllenhal is frequently encountered in cemeteries and both adults and larvae are found on buried bodies in coffins, where it may feed on phorid larvae associated with buried bodies (Blair 1922, Bousquet 2010).
Feeding habits of monotomids are not well known. Bactridium, Monotoma and Hesperobaenus are thought to be mycophagous, feeding on ascomycetes such as Hypoxylon and Daldinia (Lawrence 1977, Chandler 1983, Lawrence et al. 1999b). The adults are typically found in fruitings of these fungi, or in areas where such fungi are abundant. It is thought that most bark-inhabiting genera are mycophagous as well, though this has not been well-established most monotomid genera. Some species of Rhizophagus and Mimemodes are known to be egg predators of scolytine bark beetles, and one particular species, Rhizophagus grandis, has been released in biocontrol programs of Dendroctonus bark beetles in Europe and North America (Gregoire et al. 1985). However, species in these genera are also known to feed on fungal byproducts occurring within the galleries. Rhizophagus bipustulatus, R. dispar, and R. brunneus are known to carry Ceratocystis spp., and Hinds (1972) showed R. brunneus to be an important vector of five species of Ceratocystis in aspens.
Crowsonius similis, C. meliponae & C. parensis (Pakaluk & Slipinski 1993, Pakaluk & Slipinski 1995) are inquilines within nests of Trigona bees in Central and South America, and appear to feed on pollen and scavenge in the refuse piles of the nests. This genus may be unique among monotomids in utilizing phoresy for dispersal (Pakaluk & Slipinski 1993), a behavior that is reflected by various anatomical modifications (e.g., mandibular form, extremely reduced eyes and winglessness).
COLLECTING
Monotomidae are most often collected by sifting decaying organic matter, such as leaf litter, compost, or other fungusy material. Several species are readily attracted to blacklight traps, and many are also caught at flight-intercept and Malaise traps. Baits and pheromone traps used to attract scolytine bark beetles often catch Rhizophagus species as well. Large numbers of the North American Pycnotomina cavicolle Horn are attracted by molasses-baited traps in forested areas. Peeling bark and examining scolytine galleries of a variety of trees (see above) may yield a great diversity of monotomid genera. Sifting beach wrack can yield specimens of the coastal species in large numbers.
Characteristics
Morphology, Adults
Adults of Monotomidae are generally small (~1.5-6.0 mm) and exhibit a variety of cryptic color combinations, such as dark brown, red-brown, light brown, yellow, or black. Rhizophagus spp. often exhibit orange patches or spots. Europs spp. may have dark patches or infuscations on the elytra. Most are flattened or sub-cylindrical and are glabrous, sub-glabrous, or bear short, sparse setae on the pronotum and elytra.
Although there are many atypical genera with widely varying forms, all Monotomidae share a few basic chacteristics that can be used to distinguish them from closely related or similar-looking families. The adults can be characterized by the following combination of features (Bousquet 2010, Bousquet 2001, Sen Gupta 1988):
- generally narrow-elongate
- head prognathous, not concealed, with lateral, finely faceted eyes
- elytra distinctly truncated at the apex, exposing one (females) or two (males) abdominal tergites
- antennae short, stout, appearing 10-segmented (antennomere 10 and 11 fused) with a distinct 1 or 2 segmented club
- tarsal formula 5-5-5 in females and 5-5-4 in males (or 5-5-5 or 4-4-4 in both sexes)
- abdomen with 5 ventrites, ventrites 1 & 5 elongate, 2-4 comparatively short, subequal
- procoxae rounded with hidden trochantins in most groups, transverse with partly exposed trochantins in Rhizophagus
- procoxal cavities broadly closed externally
Morphology, Larvae
The larval form has been described for very few monotomid species. Currently 29 genera are completely unknown in the immature stages. According to Lawrence (1991), only Rhizophagus, Monotoma, Bactridium, Hesperobaenus, Shoguna, and Lenax include at least one species that has been described in the larval stage. The known rhizophagid larvae can be characterized by the following features (Bousquet 2010, Bousquet 2001, Sen Gupta 1988, Lawrence 1991):
- body elongate, subcylindrical to slightly flattened, parallel-sided or somewhat fusiform, usually granulate or tuberculate, usually with scattered, long, simple setae
- head narrower than thorax, not concealed from above
- epicranial stem absent, frontal arms U-shaped, distinctly separated at base
- ventral epicranial ridges present
- stemmata usually 1-4 on each side, occasionally absent
- frontoclypeal suture absent, labrum free
- antenna 3-segmented, with sensorium on antennomere 2
- mandibles symmetrical, with incisor edge smooth or serrate, with accessory ventral process and a narrow, simple or serrate prostheca
- maxillary palp 3-segmented
- mala falciform
- labial palps 1 or 2-segmented
- pronotum not longer than meso- and metathorax combined, slightly narrower than mesothorax
- thoracic legs 5-segmented including pretarsus
- pretarsus acute and claw-like, with two setae lying side by side
- thoracic and abdominal spiracles annular-biforous
- abdomen 10-segmented, terga I-IX often with rows of asperities, sometimes with 2-3 large palmate tubercles bearing 1-2 long setae along lateral margins or complex processes directed posteriorly
- tergum IX with a pair of fixed, usually complex urogomphi
- anal hooks absent
Taxonomy
This family has long been referred to as Rhizophagidae Redtenbacher 1845, but Pakaluk et al. (1994) acknowledged the priority of the name Monotomidae Laporte, 1840 (Bousquet 2010). Early classifications treated the genus Rhizophagus as a distinct group within the Nitidulidae, while the rest of the monotomids (e.g. Monotomini, Europini) were treated as another distinct group within the Cucujidae. Authors consistently pointed out the similarities between the two groups, but it was not until Crowson (1952) that the two groups were united into a single family. He established four subfamilies: Rhizophaginae for Rhizophagus, Lenacinae for Lenax, Thioninae for Thione and Shoguna, and Monotominae for the remaining genera. Arnett (1962) classified the now-separate family Smicripidae and the genus Hypocoprus (Cryptophagidae) as two North American monotomid subfamilies while maintaining the other four subfamilies of Crowson. Sen Gupta (1988) excluded Smicripidae and Hypocoprus from Monotomidae, and classified the remaining taxa into only two subfamilies: Rhizophaginae for Rhizophagus, and Monotominae for the remaining groups. The Monotominae were divided into four tribes: the Monotomini (Monotoma), Thionini (Thione & Shoguna), Lenacini (Lenax), and a new tribe Europini for the remaining genera. This classification was subsequently followed by Lawrence & Newton (1995), Bousquet (2001), and Bouchard et al. (2011). Kirejtschuk and Azar (2009) added the fossil subfamily Rhizophtominae to accommodate the monotypic fossil Rhizophtoma elateroides, but the subfamily was reduced to tribal rank within the Monotominae by Bouchard et al. (2011).
Discussion of Phylogenetic Relationships
Currently, the sister taxon to Monotomidae remains unclear. Crowson (1955), based on adult morphological characters, suggests a close relationship with Nitidulidae and the closely allied Kateretidae and Smicripidae. However, larval characters support a closer relationship to other primitive cucujoids such as Cryptophagidae (Lawrence 1991). Leschen et al. (2005) propose a close relationship between monotomids and the protosphindid clade, based on adult and larval characters. Using molecular data, Hunt et al. (2007) place monotomids in an “Erotylid Series” with erotylids, protocucujids and helotids. This group was recovered as the sister group to a “Nitiduloid lineage.” Robertson et al. (2008) placed monotomids among a basal cucujoid lineage that included nitiduloids. Lawrence et al. (2011) placed the Monotomidae in a basal cucujoid clade containing similar families to Leschen et al. (2005). Robertson (unpublished dissertation) conducting an in-depth DNA phylogenetic analysis of Cucujoidea using 9 genes and 341 taxa, recovered Monotomidae as sister-group to the nitiduloid lineage. In all analyses using more than one monotomid taxon, the family has been recovered as monophyletic, although only Lenax, Bactridium, Rhizophagus, Monotoma, and Monotomopsis have been included in these analyses.
Relationships among the subfamilies, tribes, and genera of Monotomidae have never been analyzed in any large-scale molecular or morphological analysis. It is likely that several of the genera are polyphyletic as they currently stand (Bousquet 2010), and a rigorous analysis at the subfamilial, tribal, and generic level is needed for taxonomic resolution.
References
Arnett, R.H. 1962. The Beetles of the United States (A Manual for Identlfication). Part V. Suborder Polyphaga (cont.); series Cucujiformia (cont.): Tenebrionoidea: Cucujoidea. 645-850. The Catholic University of America Press, Washington, D.C.
Beaver, R.A. 1966: Notes on the fauna associated with elm bark beetles in Wytham Wood, Berks. I. Coleoptera. The Entomologist's Monthly Magazine 102:163-170.
Blair, K.G. 1922. Notes on the life-history of Rhizophagus parallelocollis Gyll. The Entomologist's Monthly Magazine 58:80-83.
Bouchard P, Bousquet Y, Davies AE, Alonso-Zarazaga MA, Lawrence JF, Lyal CHC, Newton AF, Reid CAM, Schmitt M, Ślipiński SA, Smith ABT. 2011. Family-group names in Coleoptera (Insecta). ZooKeys 88: 1–972. doi: 10.3897/zookeys.88.807
Bousquet, Y. 1990. A review of the North American species of Rhizophagus Herbst and a revision of the Neararctic members of the subgenus Anomophagus Reitter (Coleoptera: Rhizophagidae). The Canadian Entomologist 122:131-171.
Bousquet, Y. 2001. 79. Monotomidae Laporte 1840. Pp. 319-321 in Arnett, R.H., Jr., Thomas, M.C., Skelley, P.E. & Frank, J.H. (eds.) American Beetles. Volume 2. Polyphaga: Scarabaeoidea through Curculionoidea. CRC Press, Boca Raton (Florida).
Bousquet, Y. 2002. Review of the genus Hesperobaenus LeConte (Coleoptera: Monotomidae) of America, north of Mexico. The Pan-Pacific Entomologist 78:197-214.
Bousquet, Y. 2010. 10.8. Monotomidae Laporte, 1840. pp. 319-324 in R.A.B. Leschen & R.G. Beutel, eds., Handbook of Zoology – Coleoptera, vol. II. De Gruyter, Berlin, New York.
Bousquet, Y. & Laplante, S. 2000. Taxonomic review of the Canadian species of the genus Monotoma Herbst (Coleoptera, Monotomidae). Proceedings of the Entomological Society of Ontario 130:67-96.
Chandler, D.S. 1983. Larvae of wrack Coleoptera in the families Corylophidae, Rhizophagidae, and Lathridiidae. Psyche 90:287-296.
Crowson, R.A. 1952. The classification of the families of British Coleoptera(cont.). The Entomologist's Monthly Magazine 88:121-132.
Crowson, R.A. 1955. The Natural Classification of the Families of Coleoptera. 187 pp. Nathaniel Lloyd, London.
De Marzo L. 2002. Larve di coleotteri in detriti vegetali di origine agricola: lineamenti morfologici e presenza stagionale (Polyphaga: 20 famiglie). Entomologica 34:65-131.
Doyen, J.T. 1976. Marine beetles (Coleoptera excluding Staphylinidae). pp. 497-519 in Cheng, L. (ed.) Marine Insects. North-Holland Publishing Co., Amsterdam.
Gregoire J.C., Merlin, J., Pasteels, J.M., Jaffuel, R., Vouland, G. & Schvester, D. 1985. Biocontrol of Dendroctonus micans by Rhizophagus grandis Gyll. (Col., Rhizophagidae) in the Massif Central (France): a first appraisal of the mass-rearing and release methods. Zeitschrift fur angewandte Entomologie 99:182-190.
Hanson, H.S. 1937. Notes on the ecology aud control of pine beetles in Great Britain. Bulletin of Entomological Research 28:185-236.
Hayashi, N., A. Fukuda, and K. Kurosa. 1959. Coleoptera, pp. 392-545. in T. Esaki, K. Yuasa, T. Ishii and T. Motoki (eds), Illustrated Insect Larvae of Japan. Hokuryukan, Toyko.
Hayashi, N. 1980. Illustrations for identification of larvae of the Cucujoidea (Coleoptera) found living in dead trees in Japan. Memoirs of the Educational Institute for Private Schools in Japan 72:95-147, 53 pls.
Hayashi, N. 1981. Illustrations for identification of the Coleopterous larvae living in dead trees. Memoirs of the Educational Institute for Private Schools in Japan 61:83-96, 12 pls.
Hayashi, N. 1992. Illustrations for identification of larvae of the superfamily Cucujoidea (Coleoptera) found in mouldy stored foods in Japan. Kaoku gaichu 14:102-131.
Hetschko, A. 1930. Fam. Cucujidae. pp. 1-122 in Junk, W. & Schenkling, S. (eds.) Coleopterorum catalogus. Pars 109. Junk, Berlin.
Hinds, T.E. 1972. Insect transmission of Ceratocystis species associated with aspen cankers. Phytopathology 62:221-225.
Hinton, H.E. and L.H. Ancona. 1935. Fauna de Coleopteros en nidos de hormigas (Atta), en Mexico y Centro-America II. Anales del Instituto de Biologia de la Universidad Nacional de Mexico. 6:307-316.
Hunt, T., Bergsten, J., Levkanicova, Z., Papadopoulou, A., John, O.S., Wild, R., Hammond, P.M., Ahrens, D., Balke, M., Caterino, M.S., Gomez-Zurita, J., Ribera, I., Barraclough, T.G., Bocakova, M. Bocak, L., Vogler, A.P. 2007. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science 218:1913-1916.
Kirejtshuk, A.G., D. Azar, P. Tafforeau, R. Boistel & V. Fernandez. 2009. New beetles of Polyphaga (Coleoptera, Polyphaga) from Lower Cretaceaus Lebanese amber. Denisia 26, zugleich Kataloge der oberösterreichischen Landesmuseen Neue Serie 86:119–130
Kishi, Y. 1970. Mimemodes japonicus Reitter (Coleoptera:Rhizophagidae), an egg predator of the pine bark beetle, Cryphalus fulvus Niijima (Coleoptera:Ipidae). Kontyu 38(3):195-197.
Klimaszewski, J. & Watt J.C. 1997. Coleoptera: Family Group Review and Keys to Identification. Fauna of New Zealand 37:1-199.
Lawrence, J.F. 1977. Coleoptera associated with an Hypoxylon species (Ascomycetes: Xylariaceae) on oak. The Coleopterists Bulletin 31:309-312.
Lawrence, J.F. 1982. Coleoptera. pp. 482-553 in Parker, S.P. (ed.) Synopsis and Classification of Living Organisms. Vol. 2. McGraw-Hill, New York.
Lawrence, J.F. 1989. Mycophagy in the Coleoptera: feeding strategies and morphological adaptations. pp. 1-23 in N. Wilding, N.M. Collins, P.M. Hammond, J.F. Webber (eds.) Insect-fungus interactions. Academic Press.
Lawrence, J.F. 1991. Rhizophagidae (Cucujoidea) (including Monotomidae). pp. 460-462 in Stehr, F.W. (ed.) Immature Insects. Volume 2. Kendall/Hunt Publishing Co., Dubuque, Iowa.
Lawrence, J. F. & E. B. Britton. 1991. 35: Coleoptera: 64. Rhizophagidae in Insects of Australia, Vol. 2. CSIRO. Ithaca, NY, Cornell University Press.
Lawrence, J.F., Hastings, A.M., Dallwitz, M.J., Paine, T.A. & Zurcher, E.J. 1999a. "Beetle Larvae of the World: Descriptions, Illustrations, Identification, and Information Retrieval for Families and Subfamilies." CD-ROM, Version 1.1 for MS-Windows. CSIRO Publishing, Melbourne.
Lawrence, J.F., Hastings, A.M., Dallwitz, M.J., Paine, T.A. & Zurcher, E.J. 1999b. "Beetles of the World: A Key and Information System for Families and Subfamilies." CD-ROM, Version 1.0 for MS-Windows. CSIRO Publishing, Melbourne.
Lawrence, J. F. & Newton, A. F., Jr. 1995. Families and subfamilies of Coleoptera (with selected genera, notes, references and data on family-group names). pp. 779-1006 in Pakaluk, J. & Slipinski, S.A. (eds.) Biology, Phylogeny, and Classification of Coleoptera. Muzeum i Instytut Zoologii PAN, Warszawa.
Lawrence, J.F., Slipinski, S.A., Seago, A.E., Thayer, M.K., Newton, A.F. & Marvaldi, A.E. 2011. Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Annales Zoologici (Warszawa) 61(1):1-217.
LeConte, J.L. & Horn, G.H. 1883. Classification of the Coleoptera of North America. Smithsonian Miscellaneous Collections No. 507:i-xxviii, 1-567.
Leschen, R.A.B., Lawrence, J.F. & Slipinski, S.A. 2005. Classification of basal Cucujoidea (Coleoptera: Polyphaga): cladistic analysis, keys and review of new families. Invertebrate Systematics 19:17-73.
Mequignon, A. 1914. Fam. Rhizophagidae. pp. 1-16 in Junk, W. & Schenkling, S. (eds.) Coleopterorum Catalogus. Pars 61. Berlin.
Miller, M.C., Moser, J.C., McGregor, M., Gregoire, J.C., Baisier, M., Dahlsten, D.L. & Werner, R.A. 1987. Potential for biological control of native North American Dendroctonus beetles (Coleoptera: Scolytidae). Annals of the Entomological Society of America 80:417-428.
Moser, J.C. 1989. Inoculative release of an exotic predator for the biological control of the Black Turpentine Beetle. pp. 189-200 in Kulhavy, D.L. & Miller, M.C. (eds.) Potential for Biological Control of Dendroctonus and Ips Bark Beetles. Stephen F. Austin State University, Nacogdoches (Texas).
Nikitsky, N.B. 1986. Beetles of the subfamilies Monotominae and Thioninae (Coleoptera, Rhizophagidae) from the Far East of the USSR. Zoologicheskii Zhurnal 65:1622-1629 [in Russian].
Pakaluk, J. & Slipinski, S.A. 1993. A new genus and two new species of Neotropical Rhizophagidae (Coleoptera) from Trigona (Hymenoptera: Apidae) nests, with a review of rhizophagid biology. The Coleopterists Bulletin 47:349-358.
Pakaluk, J. & Slipinski, S.A. 1995. Crowsonius parensis, a new species of Neotropical Rhizophagidae from the nest of the stingless bee Trigona dallatorreana Friese (Hymenoptera: Apidae) (Coleoptera: Cucujoidea). Genus (Wroclaw) 5:337-340.
Pakaluk, J., Slipinski, S.A. & Lawrence, J.F. 1994. Current classification and family-group names in Cucujoidea (Coleoptera). Genus (Wroclaw) 5:223-268.
Pal, T.K. 1996. Indoleptipsius ushae, a new genus and species of Rhizophagidae (Coleoptera: Cucujoidea) from Arunachal Pradesh, India. Hexapoda (Insecta Indica) 8:1-7.
Peacock, E.R. 1977. Coleoptera Rhizophagidae. Handbooks for the ldentification of British Insects. Vol. V, part 5(a). Royal Entomological Society of London, London. 19 pp.
Pototskaya, V.A. 1977. The larval morphology of some species of the genus Rhizophagus Hbst. and the systematic position of this genus based on the study of larval features. pp. 65-79 in Pravdin, F.N. (ed.) Stemboring Insects and their Entomophages. Nauka, Moscow [in Russian].
Robertson, J.A., M.F. Whiting and J.V. McHugh. 2008. Searching for natural lineages within the Cerylonid Series (Coleoptera: Cucujoidea). Molecular Phylogenetics and Evolution 46 (1): 193-205.
Robertson, J. A., S.A. Slipinski, K.B. Miller, M.F. Whiting & J.V. McHugh. 2010. Host Preference and Phylogeny of Cucujoidea (Coleoptera). Dissertation. Athens: Dept. of Entomology, University of Georgia.
Sen Gupta, T. 1988. Review of the genera of the family Rhizophagidae (Clavicornia: Coleoptera) of the world. Memoirs of the Zoological Survey of India, vol. 17. 58 pp. (+24 pl.).
Sen Gupta, T. & Pal, T.K. 1995. Further observations on the family Rhizophagidae with descriptions of seven new genera (Coleoptera, Rhizophagidae). Mitteilungen aus dem Zoologischen Museum in Berlin 71:129-146.
Title Illustrations

About This Page
Yves Bousquet

Agriculture Canada Biosyst. Research Inst.
Joseph V. McHugh

University of Georgia, Athens, Georgia, USA
Correspondence regarding this page should be directed to Thomas McElrath at , Yves Bousquet at , and Joseph V. McHugh at
Page copyright © 2012 , Yves Bousquet, and Joseph V. McHugh
 Page: Tree of Life
Monotomidae. Root-eating beetles.
Authored by
Thomas McElrath, Yves Bousquet, and Joseph V. McHugh.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Monotomidae. Root-eating beetles.
Authored by
Thomas McElrath, Yves Bousquet, and Joseph V. McHugh.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 06 December 2012
- Content changed 06 December 2012
Citing this page:
McElrath, Thomas, Yves Bousquet, and Joseph V. McHugh. 2012. Monotomidae. Root-eating beetles. Version 06 December 2012 (temporary). http://tolweb.org/Monotomidae/9147/2012.12.06 in The Tree of Life Web Project, http://tolweb.org/















 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site