Japetella diaphana
Richard E. YoungIntroduction
Japetella diaphana reaches about twice the length of Bolitaena pygmaea and has larger eyes. The third arms of the male have slightly enlarged suckers, but no trace of a ligula has been found. No fully mature male, however, has ever been captured.
Brief diagnosis:
A bolitaenid ...
- without a hectocotylus (ie, neither arm III with a ligula).
- with relatively large eyes on short optic stalks.
Characteristics
- Arms
- Arms III exhibit sexual dimorphism with suckers slightly enlarged on the distal 2/3rds of the arms in males. Near sexual maturity suckers on right arm III of males become abruptly enlarged (5th sucker ca 2x height of sucker 4 - see photograph); Hectocotylization (i.e., modification of the arm tip) unknown.
- Eyes
- Adjacent to brain in young animals but slightly removed in older animals (optic stalks short).
- Eyes large compared to Bolitaena of comparable size.
- Head
- Beaks: Descriptions can be found here: Lower beak; upper beak.
- Beaks: Descriptions can be found here: Lower beak; upper beak.
Comments
The nearly mature male that was examined had 4 enlarged suckers on arm III but the remainder of the arm was badly damaged during capture so details are unknown. Suckers on the left arm III were normal but other arms were damaged.
Japetella diaphana has iridophores in the skin, overlying the dorsal arms and head that lie in a plane transverse, rather than parallel, to the body axis. The function of these unusually positioned iridophores is unknown.



Figure. Dorsal view of the head and arms of a large J. diaphana, Hawaiian waters (left), (photograph by R. Young) and a side view of a small J. diaphana, Gulf of Mexico (right), (photograph by Danté Fenolio). The light is hitting the head at the correct angle to show the strong reflection (gold - left, bluish-white - right) of the iridophores.
Life history
Near sexual maturity, irridescence of the digestive gland and eyes are lost (Young, 1972) and pigmentation increases but not as greatly as in Bolitaena pygmaea. The increase in salivary gland size found in male Bolitaena pygmaea has not been documented in this species. We have seen a nearly mature male, however, that had a salivary gland over twice the size of a female of the same size. In addition the male salivary gland, in the fresh octopus, was a pearly, opaque white unlike the normal translucent appearance of the gland of subadults. Females develop a ring-shaped light organ around the mouth. Gravid females are found at the lower end of the vertical distribution range near 1000 m (nothing is known of the vertical distribution of near-mature and mature males) and brooding females near 800 m off Hawaii.

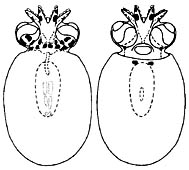
Figure. Dorsal and ventral views of small paralarva of J. diaphana, 3.8 mm ML. Drawing from Hochberg, et al., 1992.
Young are found between about 200 and 300 m or at much greater depths. Hatchlings have a ML of about 3 mm and eggs are about the same size (pers. obs.). The young paralarvae at the right have a different chromatophore pattern than that of Bolitaena pygmaea young. Unfortunately, the chromatophores are often lost during capture. Indeed, the smaller Japetella hatchling on the family page shows two posterior mantle chromatophores that apparently were lost from the paralarva illustrated here.
The life history appears to be very similar to that of Bolitaena pygmaea. Present data suggest the following senario:
Spawned females float at a depth of around 800m and brood clusters of interconnected eggs between their arms. At this depth downwelling sunlight is too low to reveal their presence; this is about as close as they can get to the surface under this visibility constraint. Females probably do not feed while brooding and die after the young hatch. At a temperature of 4-5°C, brooding is expected to take at least several months. Hatchlings either swim to the upper 200-300 m where food presumably is more plentiful or are carried there by the female at the time of hatching. Young Japetella descend into mesopelagic depths as they grow but the size at which they descend is variable (ca. 7-20mm ML). At maturity the female, and presumably the male, descends to depths of just over 1000 m where males perhaps first attract females then vice-versa. Secretions from the posterior salivary gland of males could act as a pheromone to attract females. Presumably as the female draws close and when she considers conditions safe, she will signal the male with the oral light organ and mating will occur.


Figure. Female diaphana brooding embryos. Left - Side view. Note light area (egg mass) within the center of the arm crown. Right - Same individual from a posterioventral view with arms flared enough to expose the egg mass (arrow). Photographs taken from an MBARI ROV at 910 m. © 2013 MBARI.
Distribution
Vertical distribution
In Hawaiian waters, Young (1978) found the distribution pattern shown on the right. Small octopods (ca. 5-20 mm ML) were found near 170-270 m depth or mostly between 500 and 800 m. At a ML of over 20 mm, most were captured at depths of 700-950 m. Two gravid females were captured at about 1050 m. No mature males were taken. Three brooding females were taken between 725 and 800 m.

Figure. Vertical distribution chart of J. diaphana, Hawaiian waters. Bars - Fishing range of opening/closing trawl; absence of bar indicates capture is from an open trawl. Circles - Modal depth of the trawl. Open yellow circles - day captures. Filled blue circles - night captures. Red dots with yellow cross-bars - Brooding female, day capture. Red dots with blue cross-bars - Brooding female, night capture. Red dot with green ring - Gravid female. Chart modified from Young, 1978.
The distribution seen here is similar to that found by Lu and Clarke (1975) for J. diaphana in the tropical Eastern North Atlantic except that these authors record several daytime captures of large octopods at shallow depths. Two of these captures were extreme: One was in the upper 100 m (60 mm ML) and the other in the 100-200 m range (70 mm ML). Neither octopus was mature. At present, we have no explanation for this peculiar aspect of their vertical distribution.
Geographical distribution
Japetella diaphana is found throughout the tropical and subtropical regions of the world's oceans but extends into boreal waters in the North Pacific (Thiele, 1949, Nesis, 1982).
References
Clarke, M. R. and C. C. Lu. 1975. Vertical Distribution of cephalopods at 18° N 25° W in the North Atlantic. Journal of the Marine Biological Association of the United Kingdom, 55 (1): 165-182.
Hochberg, F. G., M. Nixon and R. B. Toll. 1992. Order Octopoda Leach, 1818. In: Sweeney, M. J., C. F. E. Roper, K. M. Mangold, M. R. Clarke and S. v. Boletzky (eds.) "Larval" and juvenile cephalopods: A manual for their identification. Smithson. Contr. Zool., 513:1-282.
Nesis, K. N. 1982/87. Abridged key to the cephalopod mollusks of the world's ocean. 385+ii pp. Light and Food Industry Publishing House, Moscow. (In Russian.). Translated into English by B. S. Levitov, ed. by L. A. Burgess (1987), Cephalopods of the world. T. F. H. Publications, Neptune City, NJ, 351pp.
Thore, S. 1949. Investigations on the "Dana" Octopoda. Dana-Report No. 33: 1-85.
Young, R. E. 1972. Brooding in a bathypelagic octopus. Pacific Science, 26: 400-404.
Young, R. E. 1978. Vertical distribution and photosensitive vesicles of pelagic cephalopods from Hawaiian waters. Fish. Bull. 76: 583-615.
Title Illustrations

| Scientific Name | Japetella diaphana |
|---|---|
| Location | Northeast Pacific at 45.1°N, 125.9°W. 654 m depth |
| Comments | Image courtesy of the Monterey Bay Aquarium Research Institute (MBARI). You must obtain permission from MBARI to use this photo; please contact pressroom@mbari.org for further information |
| Acknowledgements | Susan Von Thun, photo editing, MBARI |
| Specimen Condition | Live Specimen |
| View | Side-oblique |
| Size | Small |
| Copyright | © 2011 MBARI |
About This Page

University of Hawaii, Honolulu, HI, USA
Page copyright © 2017
 Page: Tree of Life
Japetella diaphana .
Authored by
Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Japetella diaphana .
Authored by
Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 16 November 2016
Citing this page:
Young, Richard E. 2016. Japetella diaphana . Version 16 November 2016 (under construction). http://tolweb.org/Japetella_diaphana/20224/2016.11.16 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site