Crenarchaeota
Sue Barns and Siegfried Burggraf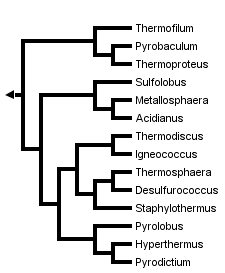

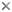
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The kingdom Crenarchaeota has the distinction of including microbial species with the highest known growth temperatures of any organisms. Although they are microscopic, single-celled organisms, they flourish under conditions which would quickly kill most "higher" organisms. As a rule, they grow best between 80° and 100°C (100°C = 212°F, the boiling point of water at sea level), and several species will not grow below 80°C. Several species also prefer to live under very acidic conditions in dilute solutions of hot sulfuric acid. Approximately 15 genera are known, and most of the hyperthermophilic species have been isolated from marine or terrestrial volcanic environments, such as hot springs and shallow or deep-sea hydrothermal vents. Recent analyses of genetic sequences obtained directly from environomental samples, however, indicate the existence of low temperature Crenarchaeota, which have not yet been cultivated.
Crenarchaeota comprise one kingdom in the larger domain of Archaea. Although they are simple, microscopic organisms, Archaea are quite distinct from more commonly encountered Bacteria, having branched off from the latter very early in evolutionary history (probably >3.5 billion years ago.) In fact, Archaea are more similar to humans than to Bacteria in many important ways, and are probably more closely related to us as well! For more information about Archaea, see Brock's Biology of Microorganisms.
Characteristics
The Kingdom Crenarchaeota has been defined phylogenetically, based on comparative molecular sequence analyses, and its members are therefore primarily defined by sequence similarity. However, like all Archaea, Crenarchaeota are prokaryotic, and are bounded by ether-linked lipid membranes which contain isoprinoid side chains instead of fatty acids. Cells range in size from cocci <1µm in diameter to filaments over 100µm in length. Species display a wide range of cell shapes, including regular cocci clustered in grape-like aggregates (Staphylothermus), irregular, lobed cells (Sulfolobus), discs (Thermodiscus), very thin filaments (<0.5µm diameter; Thermofilum), and almost rectangular rods (Thermoproteus, Pyrobaculum). Most species possess flagella and are motile. A few members of the Crenarchaeota exhibit strange morphologies: Pyrodictium produces disk-shaped cells connected by extensive networks of proteinaceous fibers which may help it to attach to sulfur granules.
Metabolically, Crenarchaeota are quite diverse, ranging from chemoorganotrophs to chemolithoautotrophs. They are anaerobes, facultative anaerobes or aerobes, and many utilize sulfur in some way for energy metabolism. Several species are primary producers of organic matter, using carbon dioxide as sole carbon source, and gaining energy by the oxidation of inorganic substances like sulfur and hydrogen, and reduction of sulfur or nitrate. Others grow on organic substrates by aerobic or anaerobic respiration or by fermentation.
The most spectacular feature of the Crenarchaeota, however, is their tolerance to, and even preference for, extremes of acidity and temperature. While many prefer neutral to slightly acidic pH ranges, members of the crenarchaeal order Sulfolobales flourish at pH 1-2 and die above pH 7. Optimum growth temperatures range from 75° to 105°C, and the maximum temperature of growth can be as high as 113°C (Pyrobolus). Most species are unable to grow below 70°C, although they can survive for long periods at low temperatures.
For more information about crenarchaeal physiology and research, check out the Web site of the laboratory of Dr. Karl Stetter, at the University of Regensburg, Germany.
Two types of environments were Crenarchaeota thrive: Left. Obsidian Pool, in the Mud Volcano area of Yellowstone National Park, is a neutral-pH hot spring which contains an unusually wide diversity of Crenarchaeota. Note yellow deposits of sulfur on banks. (Photograph by Norm Pace, © 1997.) Right. Hot, sulfur-rich, acidic habitats, like this pool in Yellowstone, are often home to species of Sulfolobus. (Photograph by S. Barns, © 1997.)
Surprisingly, recent rRNA sequence-based analyses indicate that Crenarchaeota also may be widely distributed in low-temperature environments such as ocean waters and terrestrial sediments and soils (Bintrim 1997, DeLong 1994, Furhman 1992, and Hersberger 1996). Although none of these organisms have been cultivated to date, elements of their rRNA sequences, together with the environments from which they were obtained, strongly suggest that these organisms are mesophilic (or even psychrophilic). Nothing is known of their physiology. Quantitation of rRNA abundance in Antarctic ocean waters indicates that these novel species may constitute a significant portion of the marine bacterioplankton. This, together with the fact that crenarchaeal rRNA sequences have been obtained from every low-temperature environment in which they were sought, indicates that what were once thought to be obscure organisms living in extreme conditions may instead be globally distributed, important players in the biosphere. For more information about low-temperature Crenarchaeota, have a look at the CrenPage, a Web site of Norm Pace's lab at UC Berkeley.
Crenarchaeota aren't just for microbiologists...
These unusual properties of Crenarchaeota have attracted the attention of a wide range of scientists, including evolutionary biologists, exobiologists and biotechnology companies. The extreme conditions under which Crenarchaeota live today may be similar to those which existed on the early Earth at the time that life first arose. This, together with information about their geneology, suggests that these organisms may be much like the earliest lifeforms on earth. Photographs of some regions of the surface of Mars suggest that large hot spring systems, perhaps containing microbial life, may have once existed there. As a result, NASA exobiologists may study these features for chemical and fossil remnants of organisms resembling Crenarchaeota. Finally, the extreme resistance of crenarchaeal cellular enzymes to heat and acid make them very attractive to biotechnology companies, several of which are currently developing such enzymes for industrial and research uses. To see what one biotech company, Diversa, is doing with Crenarchaeota, check out the Diversa Web site.
Discussion of Phylogenetic Relationships
Evolutionary relationships between cultivated members of the Crenarchaeota have been inferred in several studies utilizing small and large subunit ribosomal RNA sequences (Barns 1996, Kjems 1992, Burggraf 1997) Unfortunately, few sequences for genes other than rRNA from more than one crenarchaeal species have been determined to date, thus no alternative molecular sequence-based hypotheses are currently available. However, where analyses overlap, topologies of rRNA-based trees are largely concordant, and show division of the kingdom into three main lineages. The earliest branch within the kingdom contains the genera Thermoproteus, Thermofilum and Pyrobaculum, organisms distinguished by a rod-shaped morphology, neutrophily and anaerobiasis or facultative anaerobiasis. A second lineage contains the Sulfolobus, Stygiolobus, Acidianus and Metallosphaera genera, whose members share coccoid morphology and thermoacidophilic growth. The remaining Crenarchaeota cluster into a group comprised of several genera, including Pyrodictium, Desulfurococcus, Staphylothermus, Thermodiscus, Aeropyrum, Igneococcus and Thermosphaera, all of which are coccoid, neutrophilic hyperthermophiles.
Intriguingly, most of the currently available crenarchaeal rRNA sequences have been obtained from uncultivated organisms through PCR-mediated cloning and sequencing of rDNAs directly from mixed-population DNAs extracted from sediments, soils and water samples (Barns 1996, Bintrim 1997, DeLong 1994, Furhman 1992, McInerney 1995, Hershberger 1996). Most of these sequences branch more deeply from the crenarchaeal line of descent than do those of cultivated species. Addition of such sequences to phylogenetic analyses of Crenarchaeota do not substantially change apparent relationships between cultivated species. However, analysis of these environmental rDNA sequences do reveal considerably greater phylogenetic breadth than was previously known for the kingdom. To see a phylogenetic tree containing many of these environmental sequences, have a look at the Pace lab's "Cren Tree".
References
Barns, S. M., C. F. Delwiche, J. D. Palmer and N. R. Pace. 1996. Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc. Natl. Acad. Sci. USA 93: 9188-9193.
Bintrim, S.B., Donohue, T.J., Handelsman, J., Roberts, G.P., Goodman, R.M. 1997. Molecular phylogeny of Archaea from soil. Proc. Natl. Acad. Sci. USA 94:277-282.
Bloechl, E., R. Rachel, S. Burggraf, D. Hafenbradl, H.W. Jannasch and K.O. Stetter. 1997. Extremophiles 1:14-21.
Brock, T. D. 1978. Thermophilic microorganisms and life at high temperatures. New York, Springer-Verlag.
Burggraf, S., H. Huber and K. O. Stetter. 1997. Reclassification of the crenarchaeal orders and families in accordance with 16S ribosomal RNA sequence data. Int. J. System. Bacteriol., in press.
DeLong, E. F., K. Y. Wu, B. B. Prezelin and R. V. M. Jovine. 1994. Archaea in Antarctic marine environments. Nature 371: 695-697.
Fuhrman, J. A., K. McCallum and A. A. Davis. 1992. Novel marine archaebacterial group from marine plankton. Nature 356: 148-149.
Hershberger, K.L., S. M. Barns, A.-L. Reysenbach, S.C. Dawson and N.R. Pace. 1996. Wide diversity of Crenarchaeota. Nature 384:420.
Kjems, J., N. Larsen, J. S. Dalgaard, R. A. Garrett and K. O. Stetter. 1992. Phylogenetic relationships amongst the hyperthermophilic Archaea determined from partial 23S rRNA gene sequences. System. Appl. Microbiol. 15: 203-208.
McInerney, J. O., M. Wilkinson, J. W. Patching, T. M. Embley and R. Powell. 1995. Recovery and phylogenetic analysis of novel archaeal rRNA sequences from a deep-sea deposit feeder. Appl. Envir. Microbiol. 61: 1646-1648.
Rieger, G., R. Rachel, R Hermann and K.O. Stetter. 1995. J. Struct. Biol. 115:78-87.
Segerer, A. H., S. Burggraf, G. Fiala, G. Huber, R. Huber, U. Pley and K. O. Stetter. 1993. Life in hot springs and hydrothermal vents. Orig. Life Evol. Biosph. 23: 77-90.
Stetter, K. O. 1996. Hyperthermophilic procaryotes. FEMS Microbiol. Revs. 18: 149-158.
Voelkl, P., R. Huber, E. Drobner, R. Rachel, S. Burggraf, A. Trincone and K.O. Stetter. 1993. Appl. Environ. Microbiol. 59:2918-2926.
Woese, C. R. 1993. The Archaea: Their history and Significance, vii-xxviii. In M. Kates et al. (eds.), The Biochemistry of Archaea (Archaebacteria). Elsevier Science Publishers.
Zillig, W., Stetter, K.O., Schafer, W., Janekovic, D., Wunderl, S., Holz, J. and P. Palm. 1981. Thermoproteales: a novel type of extremely thermoacidophilic anaerobic archaebacteria isolated from Islandic solfataras. Zbl. Bakt. Hyg., I. Abt., Orig. C2:205-227.
Title Illustrations

| Scientific Name | Pyrodictium |
|---|---|
| Comments | Scanning electron micrograph of Pyrodictium cells, connected by a network of protein "fibers" (Rieger 1995) |
| Copyright | © 1997 R. Rachel |
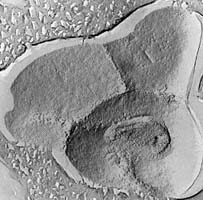
| Scientific Name | Pyrolobus |
|---|---|
| Comments | Pyrolobus, the organism with the highest known growth temperature (113°C). Transmission electron micrograph of freeze-etched cells (Bloechl 1997). |
| Copyright | © 1997 R. Rachel |
About This Page
The authors greatfully acknowledge Drs. Reinhard Rachel and Norman Pace for providing the photographs which grace these pages.
Sue Barns

Los Alamos National Laboratory, New Mexico, USA

Labor Becker, Olgemöller & Kollegen, Munich, Germany
Correspondence regarding this page should be directed to Sue Barns at
Page copyright © 1997 and
All Rights Reserved.
Citing this page:
Barns, Sue and Siegfried Burggraf. 1997. Crenarchaeota. Version 01 January 1997. http://tolweb.org/Crenarchaeota/9/1997.01.01 in The Tree of Life Web Project, http://tolweb.org/










 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site